Oxygen, an essential component of our reality, assumes a focal part on the planet as far as we might be concerned. From its revelation to its fundamental properties, different purposes, and fascinating realities, oxygen holds an extraordinary spot in the narrative of science and the science of life itself. In this article, we will investigate the revelation, image, properties, utilizes, and entrancing realities about this fundamental component.
Revelation of Oxygen:
The revelation of oxygen is frequently ascribed to different researchers, however the most eminent figure in this account is the English researcher Joseph Priestley. In the late eighteenth 100 years, Priestley directed tests that prompted the confinement of oxygen. In 1774, he produced oxygen by warming mercuric oxide and recognized it as a particular gas. Priestley’s noteworthy work made ready for the comprehension of this indispensable component.
Image and Compound Properties:
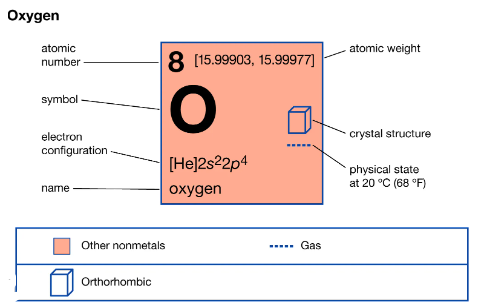
Oxygen is addressed by the compound image “O” and is the eighth component on the intermittent table. It is the most plentiful component on The planet, making up around 21% of the World’s climate. Its compound properties are characterized by its high electronegativity and its job as a strong oxidizing specialist. Oxygen promptly shapes compounds with practically any remaining components, frequently through substance responses that include the exchange of electrons. This reactivity is a key part of its importance in the regular world.
Actual Properties:
Oxygen is a diatomic gas, meaning it exists as O2 particles, and it is dull, unscented, and bland. It has an edge of boiling over of – 183 degrees Celsius (- 297 degrees Fahrenheit) and an edge of freezing over of – 218 degrees Celsius (- 361 degrees Fahrenheit). Oxygen is fundamental for ignition and breath and assumes a basic part in supporting life on The planet.
Utilizations of Oxygen:
1. Respiration: Oxygen is essential for the breath of practically all residing organic entities, where it assumes a critical part in the development of energy. It is moved in the circulation system to each cell in the body, where it helps in the breakdown of glucose to produce ATP, the cell energy money.
2. Combustion: Oxygen is essential for burning, and this property has prompted a large number of utilizations, from warming and cooking to modern cycles. It is utilized in the oxy-fuel welding and cutting industry, where it upholds high-temperature welding processes.
3. Medical Applications: Oxygen treatment is a typical clinical therapy to help patients with breathing hardships. Oxygen tanks and concentrators are utilized to give a supplemental oxygen source to those out of luck.
4. Chemical Production: Oxygen is utilized in different substance processes, including the development of steel and as an oxidizer in rocket drive.
5. Environmental Cleanup: at times, oxygen is utilized to work with bioremediation processes, where it is acquainted with tainted regions to help the development of oxygen-subordinate microbes that assist with breaking down contaminations.
Captivating Facts:
1. The World’s environment wasn’t generally oxygen-rich. It required great many long periods of photosynthesizing cyanobacteria and the development of plants to progressively build the barometrical oxygen levels to what we have today.
2. Oxygen was initially named “dephlogisticated air” by Joseph Priestley, mirroring the obsolete hypothesis of phlogiston, which was accepted to be delivered during ignition.
3. Fluid oxygen is light blue and is utilized as a rocket fuel in the airplane business.
4. Oxygen upholds fire, yet it doesn’t consume. It is the fuel (e.g., wood or gas) that consumes when presented to oxygen.
5. Oxygen is utilized to treat height affliction and is frequently conveyed by climbers and explorers in high-elevation locales.
Oxygen is, for certain, perhaps of the most pivotal component in the occasional table. Its revelation has molded how we might interpret science and the key cycles of life. From supporting our very breath to empowering endless modern applications, this diatomic gas is, straightforwardly, the breath of life on The planet.